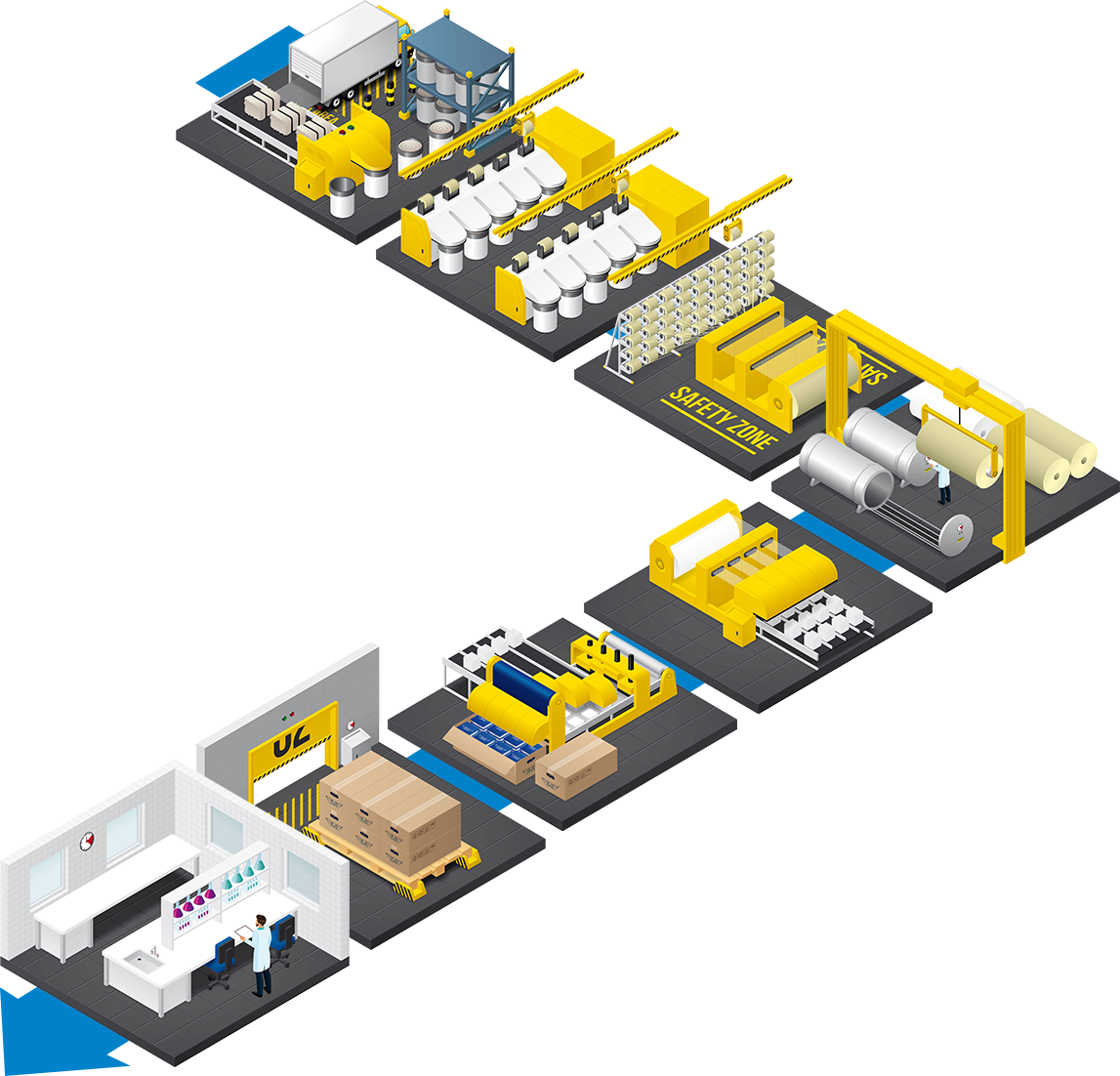
There is a long path between raw cotton and the final product shipment. The use of the best raw materials by the company ADA FIOS, the only European manufacturer of hydrophilic gauze, along with ADATEC’s state-of-the-art technology, allows ADA to focus its full potential on the production of swabs with recognized quality and certified by the competent external bodies.



